Cision PR Newswire
Navident EVO receives FDA Clearance for Guided Endodontics
TORONTO, Sept. 19, 2024 /PRNewswire/ - ClaroNav Inc, a leader in surgical navigation and photogrammetry systems, is proud to announce that it has received clearance from the U.S. Food and Drug Administration to expand the Navident EVO system's indications for use to include guided endodontics.
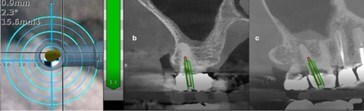
Navident EVO will apply its dynamic navigation technology to provide minimally invasive access to tooth structures requiring endodontic treatment. The dual advantage of preserving tooth structure and making the procedure more efficient significantly benefits the dentist and the patient. The advantages are clear:
- Less invasive access of calcified and hidden canals
- Preserved tooth structure
- Saved time in locating and accessing canals
"Accessing narrow canals or creating endodontic access through full coverage restorations can be a big challenge for any endodontist. Doing these procedures without Navident dynamic navigation increases the chance of error as well as the time taken for each procedure. It is a very useful technology even for experienced clinicians." says Dr. Paula Villa, from Medellin Colombia, a Global Lecturer Professor, and Founder of the university of Antio quia Endodontic Program.
Navident has been cleared for guiding dental implant treatment for 10 years. Since that time, it has expanded its protocols to include fully edentulous implant cases, endodontic treatments and other complex indications in various global regions.
"l absolutely recommend Navident EVO to my colleagues. It has added a different dimension to my practice and has made very challenging cases less time-consuming." from Chicago Illinois a Global Lecturer, DDS, MS, PhD. says Dr. Mohamed Fayed.
An Endodontic mode has been created within the Navident EVO system, improving the ability of the clinician to effectively detect, diagnose, and plan treatment prior to dynamic navigation.
"We are very proud to provide the endodontic practitioners and patients in the United States with this toothsaving and time-saving technology. The ClaroNav Dental team will continue to expand its scope of indications and its range of applications to benefit dental clinicians and their patients around the world." said Jason Pardo,.President of ClaroNav Dental.
To learn more about Navident EVO and all the ClaroNav Dental products, please visit our new website at www.claronavdental.com.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/navident-evo-receives-fda-clearance-for-guided-endodontics-302253744.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/navident-evo-receives-fda-clearance-for-guided-endodontics-302253744.html
SOURCE ClaroNav Inc.

NOTE: This content is not written by or endorsed by "WGNO", its advertisers, or Nexstar Media Inc.